Monosaccharides

They
are the simplest of the carbohydrates, the subunits from which disaccharides,
oligosaccharides and polysaccharides are constructed. They are colourless,
crystalline solids that are freely soluble in water but insoluble in
nonpolar solvents and most of them have a sweet taste.
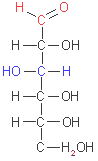
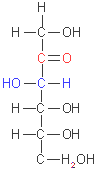
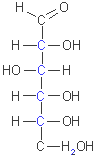
D-glucose
(aldohexose)
D-fructose (ketohexose)
Monosaccharides
have an unbranched carbon chain as a backbone, which all the carbon
atoms are linked by single bonds. One of the carbon atoms is doble-bonded
to an oxygen atom to form a carbonyl group (its position in the molecule
define if it is an aldose or a ketose); each of the other carbon atoms
has a hydroxyl group, which ones are often chiral
centres.
Assimetric
Carbon Atoms
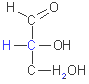
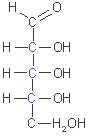
D-glyceraldehyde
D-ribose
D-glucose
The
simplest monosaccharides are the two three-carbon trioses: glyceraldehyde,
an aldose, and dihydroxycetone, a ketose. Monosaccharides with four,
five, six and seven carbon atoms in their backbones are called, respectively,
tetroses, pentoses, hexoses and heptoses.
Monosaccharides
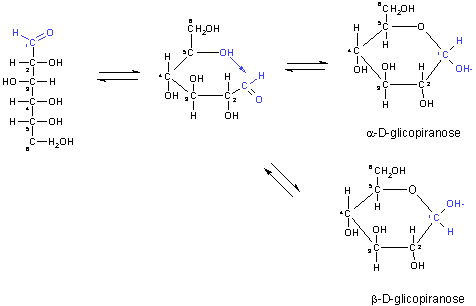
with five or more carbon atoms in the backbone usually occur in aqueous
solution as cyclic (ring) structures, in which carbonyl group has formed
a covalent bond with the oxygen of a hydroxyl group along the chain.
This reaction results in an a or ß steroisomeric, a general reaction
between aldehydes and alcohols to form derivates called hemiacetals.
Such cyclic forms of sugars are called pyranoses, when they resemble
the six-membered ring compound pyran, or furanoses, when they resemble
the five-membered ring compound furan.
Cyclic
Estructure of D-glucose
This
biomolecule group can be oxidized by relatively mild oxidizing agents
such as ferric (Fe3+) or cupric (Cu2+) ion. The carbonyl carbon is oxidized
to a carboxylic acid. Glucose and other sugars capable of reducing ferric
or cupric ion are called reducing sugars.