Oligosaccharides

Oligosaccharides are made by the joint of two or more units of monosaccharides. The disaccharides are the most common of them. Disaccharides such as maltose, lactose and sucrose consist of two monosaccharides joined covalently by an O-glycosidic bond. This formation represents the formation of an acetal form a hemicetal (glucopyranose) and an alcohol (a hydroxyl group of a second sugar molecule).
Glicosidic Bond Formation
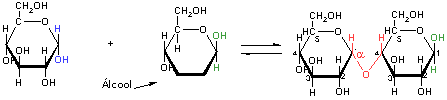
a-D-glucose
ß-D-glucose
Maltose
In blue and green are seen the hemicetal end of a-D-glucose and of a ß-D-glucose molecule participating of an acetal O-glycosidic bond (in orange) to form maltose. Make note that in the end of a chain has a free anomérico carbon (in not involved in a glycosidic bond) is commonly called the reduction end of the chain. So, maltose is a reducing sugar.
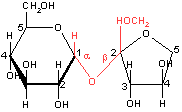
When an anomeric carbon becomes involved in a glycosidic bond, it cannot be oxidized by cupric or ferric ion. The sugar containing the anomeric carbon atom no longer acts as a reducing sugar. An example is the sugar sucrose.
Sucrose, a non-reducing sugar
Another type of glycosidic bond joints the anomeric carbon of sugar to a nitrogen atom; N-glycosyl bonds, found in all nucleotides.